General Info and Links
Unit A: Electrochemical Changes
|
Notes:
Electro Review Key:
|
Assignments:
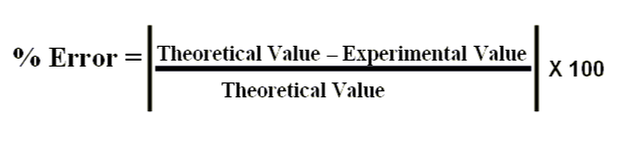
***Help for the Titration Lab write-up. Percent error is below. The original solution was 3% H2O2, and it was diluted 25 mL H2O2 in 1L solution. Use the table on the front page of your lab to help you convert to mol/L.
|
Unit B: Organic Compounds
Assignments:
Media:
|
Unit C: Thermochemical Changes
Notes: |
Assignments: |
|
% yield = actual quantity / predicted quantity *100
|